gas analysis of sample shows that it has 20h2|experimental determination of the gas : Brand In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the Gas Constant, \(R\). The single displacement reaction between magnesium metal and . Sobre a partida. Shakhtar Donetsk está enfrentando Dynamo Kyiv começando em 11 de mai. de 2024 às 12:00 UTC no Olimpiysky National Sports Complex estadio, Kyiv cidade, Ukraine. A partida faz parte do Premier League. Shakhtar Donetsk enfrentou Dynamo Kyiv em 1 partidas nesta temporada.
{plog:ftitle_list}
Resultado da On NudeLive you can sex chat with cam girls, watch free cam shows, or private chat with cam girls that will do just about anything you ask them to.Better than paid sex cam sites, our free cams allow you to watch and chat with thousands of webcam models instantly. Forget about free porn sites .
Gas analysis of sample shows that it has 20% H_2, 40% CH_4, 10% CO, 20% C_2H_6 and 10% non combustible inert gases (Volume%). 2m^3 volume of above sample is combusted (burnt) .
In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the Gas Constant, \(R\). The single displacement reaction between magnesium metal and . To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(A\) to the total pressure of a gas mixture that .Gas stoichiometry is the study of the relative amounts of reactants and products in reactions that involve gases. EXAMPLE. Calculate the volume of gaseous NO₂ produced by the combustion .Δn = 2- (3+1) = -2. Example: A mixture of nitrogen and hydrogen in a reaction vessel is allowed to attain equilibrium at 472°C. the equilibrium mixture of gases was analyzed and found to .
13-46 The volumetric analysis of a mixture of gases is given. The volumetric and mass flow rates are to be determined using three methods. Properties The molar masses of O2, N2, CO2, and .To relate the amount of gas consumed or released in a chemical reaction to the stoichiometry of the reaction. To understand how the ideal gas equation and the stoichiometry of a reaction .The volume of oxygen produced STP in litre is X. Find value of 9. Gas analysis of sample shows that it has 20% H, 40% CH4, 10% CO, 20% C,Hand 10% noncombustite inert gases .
hydrogen gas constant calculation
Chemistry. Secondary School. answered. Gas analysis of sample shows that it has 20% H_2, 40% CH_4, 10% CO, 20% C_2H_6 and 10% non combustible inert gases .Figure 3 a shows the gas response of the lettuce-like ZnO to 100 ppm H2S gas at various operating temperatures from 20 °C to 250 °C. When the temperature is lower than 50 °C, the resistance of .Soot is measured from a gas sample drawn off the exhaust flue. Draft is the differential pressure between the inside and outside of the exhaust flue. Once these measurements are made, the data is interpreted using calculated combustion parameters . Many boiler manufacturers suggest that flue gas analysis be performed at least monthly. Boiler .Assume the surroundings to be at 25C and 100kPa. Round your final answer to one decimal place. Combustion gases enter a gas turbine at 900c, 800kpa, and 103 m/s and leaves at 650C, 400kpa and 247 m/s. The molar analysis of a gas mixture at 30 o C, 2 bar is 40% N 2 , 50% CO 2 , and 10% CH 4 . Determine: (a) The analysis in terms of mass fractions.
A certain natural gas has the following volumetric analysis: 65 percent CH 4 , 8 percent H 2 , 18 percent N 2 , 3 percent O 2 , and 6 percent CO 2 . This gas is now burned completely with the stoichiometric amount of dry air. What is the air–fuel ratio for this combustion process?
The apparatus is most suitable for on-site gas analysis during field work and at remote locations due to its small size (60 cm × 40 cm × 14 cm), low weight (13 kg), and low power consumption (50 W).A combustion analysis of a 0.44g sample of an unknown compound yields 0.88 g CO_2 and 0.36 g H_2O. If the sample a molar mass of 132 g/mol, what is the molecular formula of the sample? A mixture of C_3H_8 and C_2H_2 has a mass of 2.3 g.Co ii. H2 iii. H2O iv. Sensible heat f. Thermal efficiency 1. Bottled gases are the liquefied petroleum gases propane and butane. If a sample of this gas were burned in excess air, a burner gas of the following analysis is obtained: 8.62% CO2, 1.38% CO, 6.45% O2, and 83.55% N2. Calculate: a. % excess air b. Composition of the bottled gas de 335 .many different gases, so the thermodynamic properties of a mixture result from a combination of the properties of all of the individual gas species. The ideal gas law is assumed for gaseous mixtures, allowing the ideal gas relations to be applied to each gas component. Starting with a mixture of K different gases, the total mass, m, of the .
Combustion reactions are exothermic processes where a fuel reacts with an oxidant, releasing energy in the form of heat and light. In the scenario given, we are working with a natural gas mixture that includes components like methane (CH_4), hydrogen (H_2), and other inert gases like nitrogen (N_2) and carbon dioxide (CO_2).1. A sample of dry anthracite has the following composition by mass: C 90%, H 3%. O 2.5%, N 1%, S 0.5% and ash 3%. Calculate the stoichiometric A/F ratio and determine the dry and wet analysis of the combustion products by mass and by volume wher [11.24] 20% excess air is supplied. 2. Ethyl alcohol has the following formula C2H6O. Q: The dry exhaust gas from the oil engine has the following gravimetric analysis:CO2 = 21.6% O2 = 4.2% N = 74.2%Specific heats at constant pressure for.
mass of gas V is 16.0; calculate the molecular mass of W . 3. (a) State Charles’ Law (b) The volume of a sample of nitrogen gas at a temperature of 291K and 1.0 x 10 5 Pascals. was 3.5 x 10-2 m 3.Calculate the temperature at which the volume of the gas would be. 2.8 x 10-2 m 3 at 1.0 x 10 5 pascals.. 4. 60 cm 3 of oxygen gas diffused through a porous partition in 50 seconds.Fig. 5 shows the images formed on mirror M 2.It comprises two levels. The 4n-2 and 4n (where n is a natural number) show the sequence numbers of the images formed on mirror M 2.The infrared light reflected multiple times between mirrors M 1, M 2, and M 3 is finally reflected from mirror M 3 and is focused on the exit port before reaching the detector. Fig. 6 shows the .Gas stoichiometry is the quantitative relationship between reactants and products in a gas reaction. Using PV=nRT, gas stoichiometry applies when the gases are ideal, and the temperature, pressure, and volume of the gases are all known. . If a sample of 4 moles of gas has a pressure of 567.42 kPa and a volume of 12.0 L, what is its temperature?
The chemical processes that use syngas are usually multi-stage, have a variety of gas analysis needs, and are typically dynamic, i.e., they are dependent on process gas analysis for optimization. Rapid, multi-component, .Residual fuels have more of the ash-forming constituents. These salts may be compounds of sodium, vanadium, calcium, magnesium, silicon, iron, aluminum, nickel, etc. Typically, the ash value is in the range 0.03–0.07%. Excessive ash in liquid fuels can cause fouling deposits in the combustion equipment. Ash has erosive effect on the burner tips, Based on our analysis of the security value versus the cost of implementation, we feel it’s time to add Microsoft Defender Antivirus’ Block At First Sight (BAFS) feature to the security baseline. . Join Microsoft MAPS .
A certain natural gas has the following volumetric analysis: 65 percent CH 4 , 8 percent H 2 , 18 percent N2 , 3 percent O2 , and 6 percent CO2 . This gas is now burned completely with the stoichiometric amount of dry air. What is the air–fuel ratio for this combustion process? Answer: 9.42 kg air/kg fuelThe sample syn-gas is collected in a gas sample bag and then injected into GC for analysis. Then, each sample syn-gas is injected and analyzed at least five times by the GC and the results should be averaged.. The syn-gas was produced through the GEK gasification system prior to gas analysis. Below are two multi-step procedures for gas analysis. Our biennial GAS Analysis event in English only, is the leading global symposium for gas analysis and the best forum for the latest developments and applications in industry and society. GAS Analysis attracts hundreds of experts from national and international institutions, world-renowned industry and academia, representing more than 30 countries.
Check if Windows 10 20H2 is installed with Settings. To confirm if your device has the Windows 10 20H2, use these steps: Open Settings. Click on System. Click on About. Windows 10 20H2 check with Settings; In the “About” page, under “Version”, you should see the 20H2 number, and under “OS Build”, the number should be 19042.572 or later.
A. The isotope 2H has a high natural abundance. B. 2H 2 O (s) has a higher melting point than normal ice. C. 2H 2 O (s) has a lower density than normal ice-cold water. D. 2H 2 O has different chemical properties from normal water. 3. The table lists successive ionization energies of an element Z. Ionization number 1st 2nd 3rd 4th 5th 6th1.5 Calculation of the composition of fuel and excess air supplied from the exhaust gas analysis: Some times the composition of fuel is unknown and it becomes necessary to judge whether the amount of air supplied is sufficient or not, or excess. This can be obtained by analyzing the sample of exhaust gases. Example 3Sequential smoking for smaller sample quantities: The LM5 is designed to accommodate smaller sample quantities, making it a versatile option for various applications. Multiple smoke trapping options: LM5, provides flexibility in smoke trapping options, including CFPs, Impingers, electrostatic precipitation, gas collection bags, and on-line .This document contains 5 problems related to calculating properties of gaseous fuels and their combustion products: 1. It calculates the Orsat analysis of combustion products from burning hexane with excess air. 2. It determines the gross and net calorific values of a methane-enriched gas from water gas in various units. 3. It calculates the gross and net calorific values of .
Carburetted water gas is produced in the same way as blue water gas except that is done in the presence of cracked oil vapors in a carburetor. A typical gas analysis shows 4.7% CO2 , 7.8% C2H4 , 0.3% O2, 36.5% H2, 35.5% CO, 8.6% CH4 and 6.6% N2.A typical gas analysis shows 4.7 % CO 2, 7.8 % C2H4, 0.3 % O2, 36.5 % H2, 35.5 % CO, 8.6 % CH4 and 6.6 % N2. If this gas is saturated H2O at 20°C, 742 torrs and burned in 10.434 m3 air at 30°C, 101 KPa and 60% RH per m3 fuel. . Bottled gases are the liquefied petroleum gases propane and butane. If a sample of this gas is burned in excess .
hydrogen constant lab questions
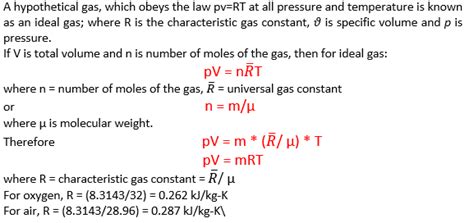
how to determine the gas constant
is the nys permit test hard
webRosiane Rosseto Borelli, Sorocaba, Brazil. 33 likes. Health/beauty
gas analysis of sample shows that it has 20h2|experimental determination of the gas